Cutting edge of diphenyl phosphorazidate (DPPA) as a synthetic reagent – A fifty-year odyssey - Vapourtec

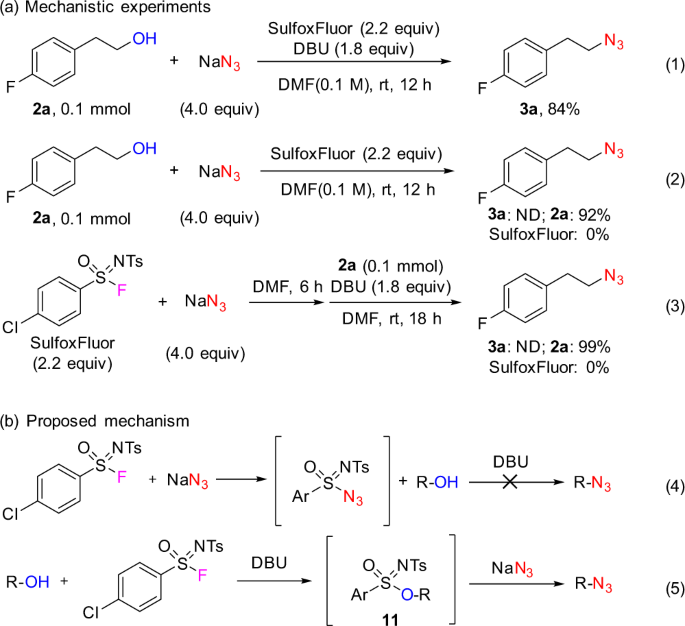
Where does the proton come from in a Mitsunobu azidation of secondary alcohol with DIAD/DPPA? | ResearchGate
Cesium carboxylates in dimethyl formamide. Reagents for introduction of hydroxyl groups by nucleophilic substitution and for inversion of configuration of secondary alcohols | The Journal of Organic Chemistry
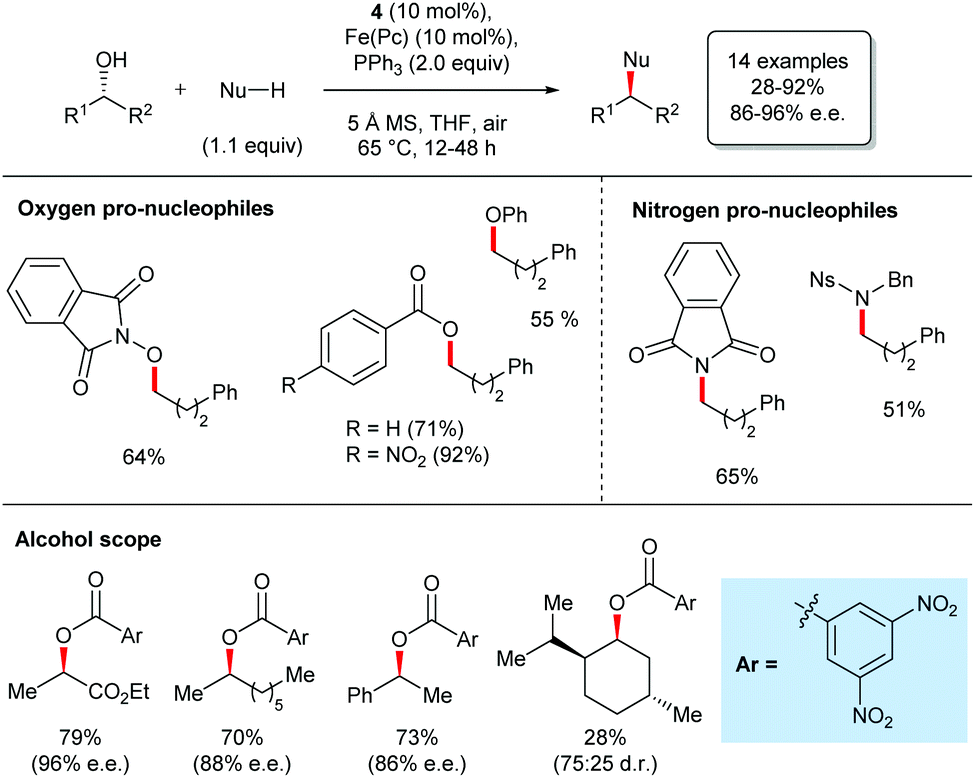
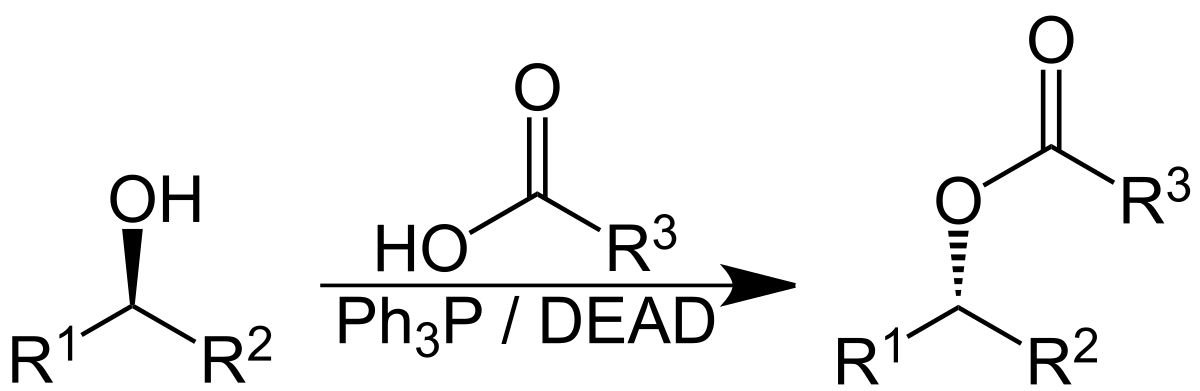
The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB01929K

An exception to the normal Mitsunobu reaction with phenols; the formation of hydrazones from salicylaldehydes - ScienceDirect

Mitsunobu Functionalization of Poly(vinyl alcohol) Derivatives† - Zhang - 2023 - Chinese Journal of Chemistry - Wiley Online Library
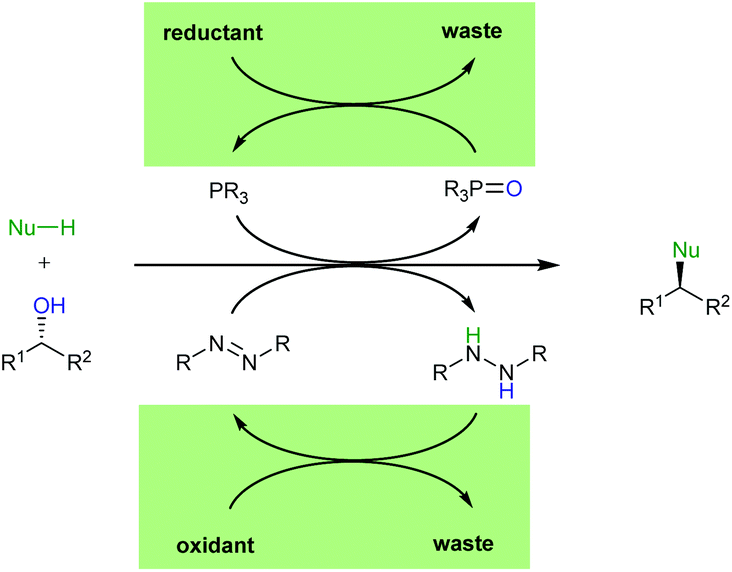
The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB01929K
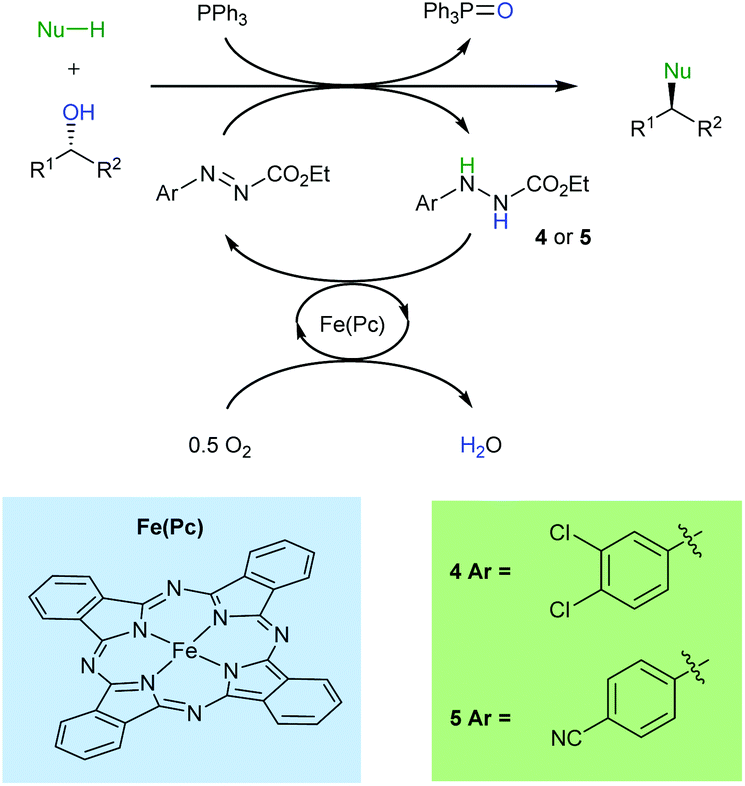
The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB01929K

PDF) Applications of the Mitsunobu reaction in peptide chemistry | Kazimierz Wisniewski - Academia.edu

Where does the proton come from in a Mitsunobu azidation of secondary alcohol with DIAD/DPPA? | ResearchGate

Preparation of Isocyanates from Primary Amines and Carbon Dioxide Using Mitsunobu Chemistry1 | The Journal of Organic Chemistry

Microwave-assisted combined Mitsunobu reaction–Claisen rearrangement and microwave-assisted phenol oxidation: rapid synthesis of 2,6-disubstituted-1,4-benzoquinone natural products - ScienceDirect

An exception to the normal Mitsunobu reaction with phenols; the formation of hydrazones from salicylaldehydes - ScienceDirect

Diversity-Oriented Approach to N-Heterocyclic Compounds from α-Phenyl-β-enamino Ester via a Mitsunobu-Michael Reaction Sequence | The Journal of Organic Chemistry

Azodicarbonyl dimorpholide (ADDM): an effective, versatile, and water-soluble Mitsunobu reagent - ScienceDirect