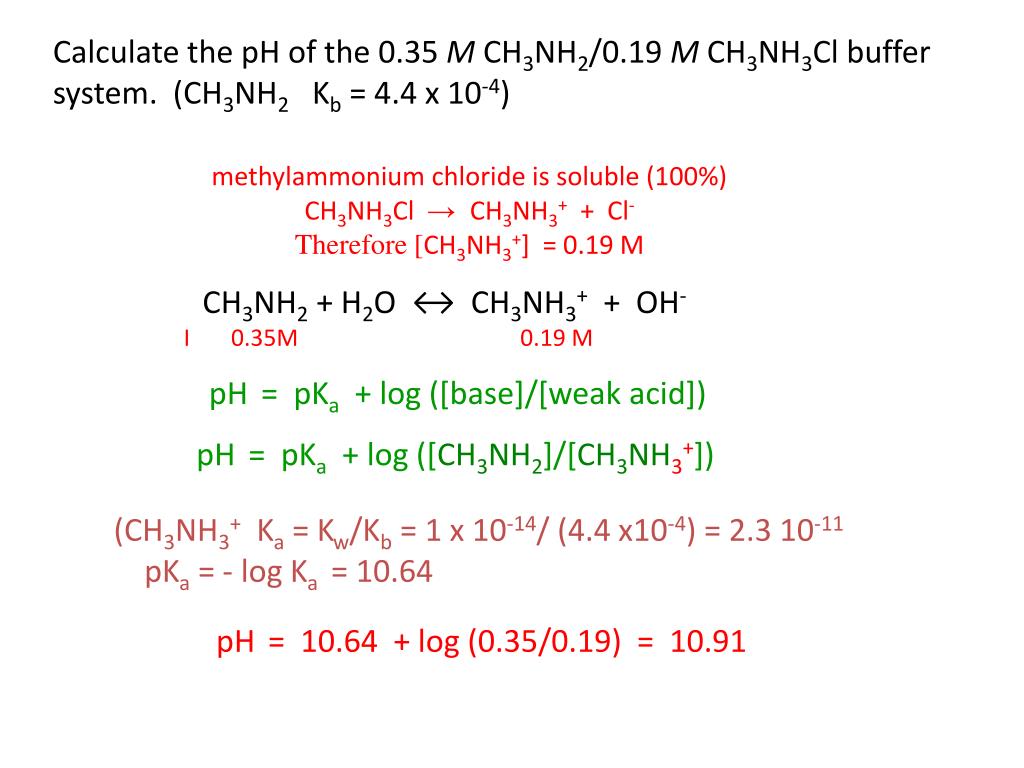
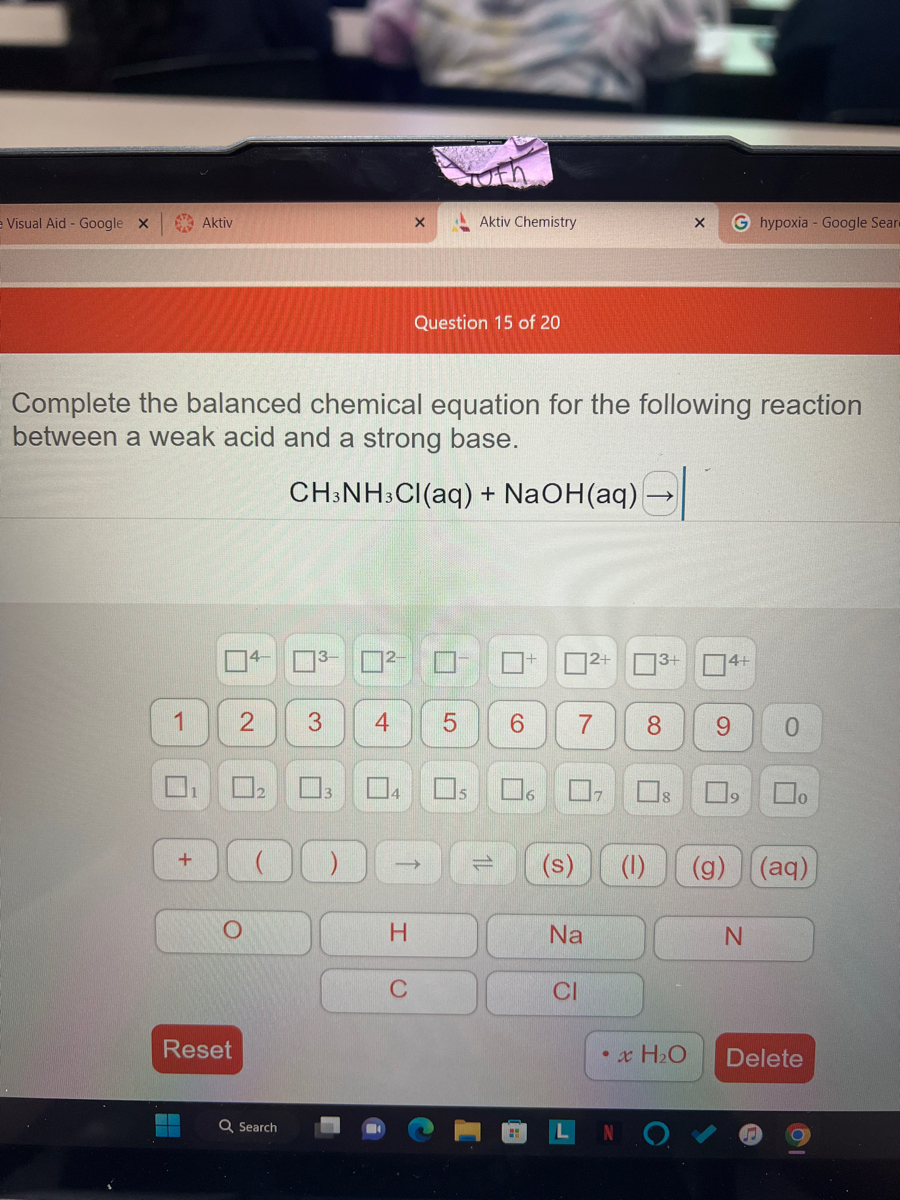
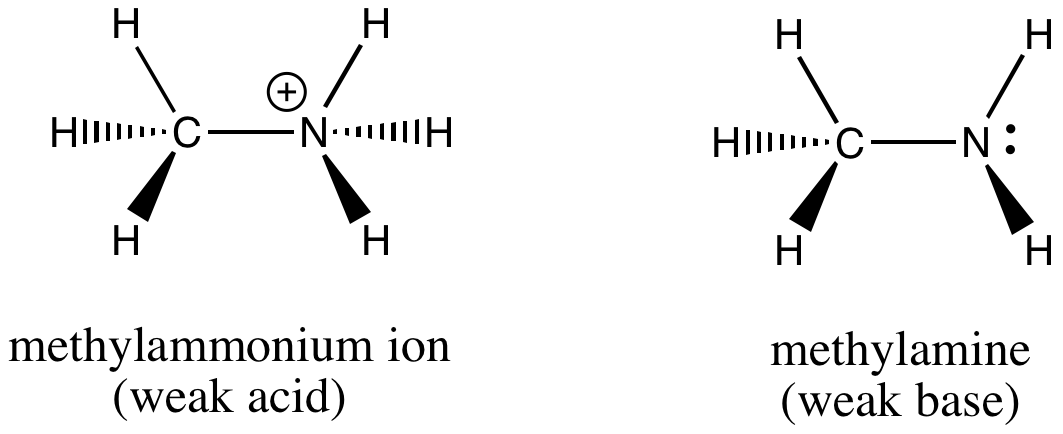
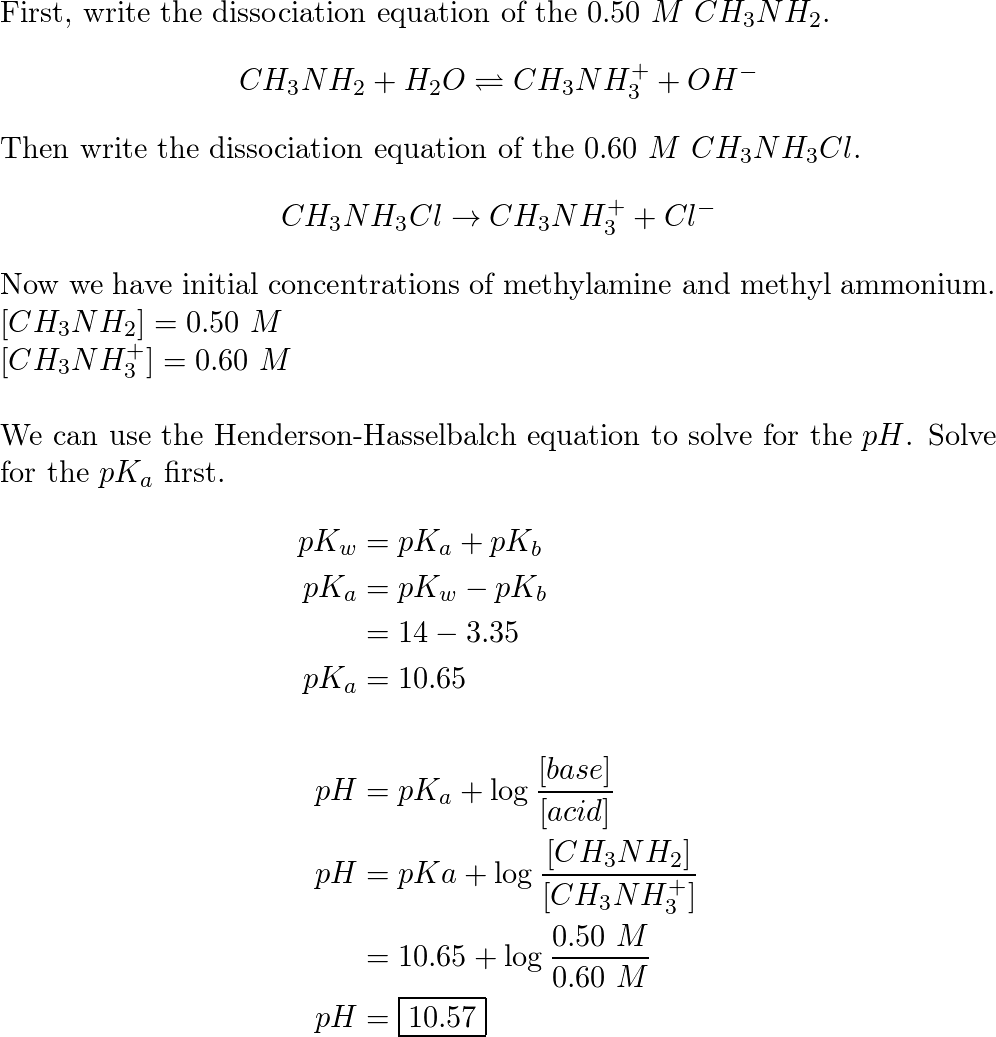OneClass: please write an EQUILIBRIUM reaction that shows CH3NH3Cl asacidic- Write a chemical equatio...

SOLVED: Methylamine, CH3NH2, and methylammonium chloride, CH3NH3Cl write the identity of the two species in each set (strong acid/strong base/weak acid/weak base/salt/other) and explain reason. Identify if anything in common betweenthe two

Solved) - Is the solution of KCI acidic, basic or neutral?. Is the solution... (1 Answer) | Transtutors

CH3NH3Cl-Assisted One-Step Solution Growth of CH3NH3PbI3: Structure, Charge-Carrier Dynamics, and Photovoltaic Properties of Perovskite Solar Cells | The Journal of Physical Chemistry C

OneClass: please write an EQUILIBRIUM reaction that shows CH3NH3Cl asacidic- Write a chemical equatio...

CNX Chemistry SSM Ch14 Mod06 - OpenStax College Chemistry 14: Buffers Chemistry 14: Acid-Base - Studocu

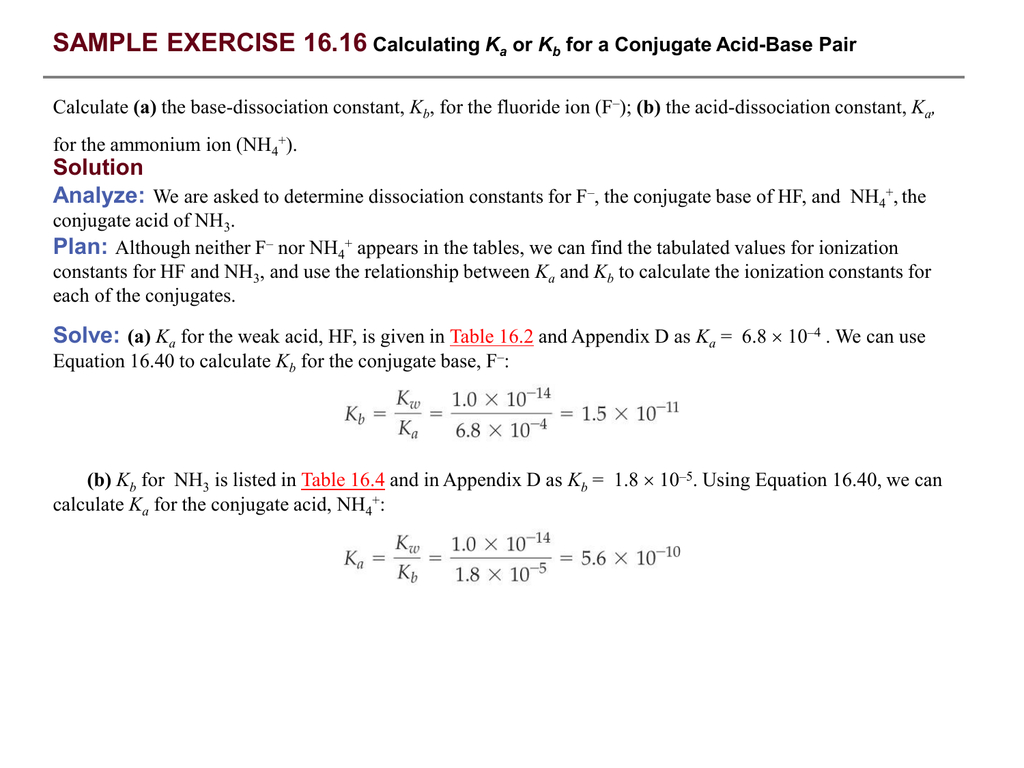
Exam 1120 .pdf - Question: Write the equilibrium-constant expressions and obtain numerical values for each constant in * a the basic dissociation of | Course Hero
How would you determine is the following salts will from a solution that is acidic, basic, or pH neutral? CH3NH3CN, Fe(ClO4)3, K2CO3, CH3NH3CL, RbI | Socratic
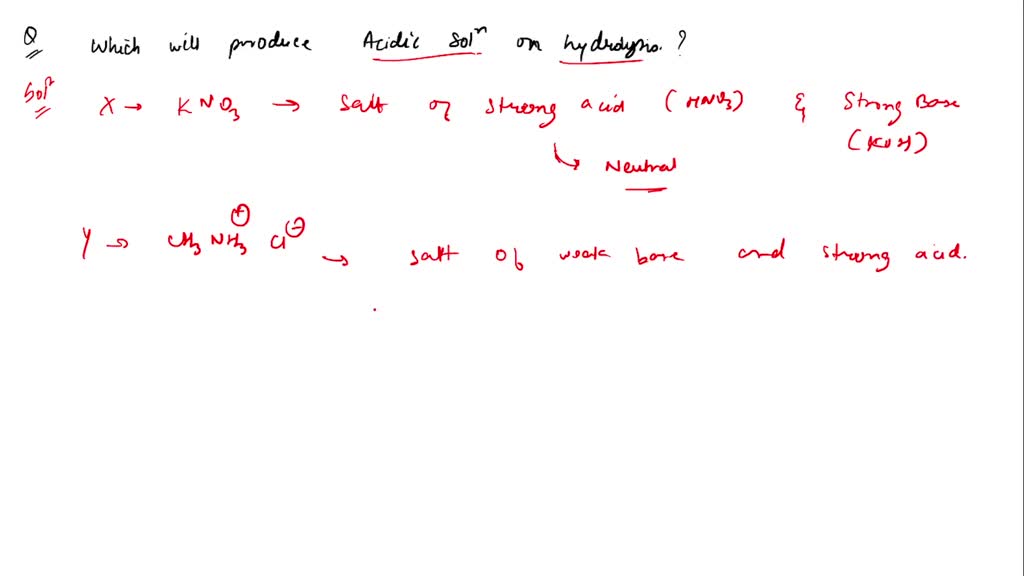
SOLVED: Which of the following compounds, when dissolved in water will produce an acidic solution? X. KNO3 Y. CH3NH3Cl Z. NaHSO4 X only X and Y Y and Z (Correct answer) Z

SOLVED: A buffer solution contains 0.50 M methylamine (CH3NH2) and 0.50 M methylammonium chloride (CH3NH3Cl), resulting in the equilibrium: CH3NH3+(aq) + H2O(l) ⇌ CH3NH2(aq) + H3O+(aq) One mL of 0.10 M HNO3